Draw the major product of the reaction sequence. omit byproducts. – Delving into the realm of reaction sequences, we embark on a journey to decipher the intricate dance of chemical transformations. By understanding the principles that govern these sequences, we gain the power to predict the major products that emerge from a series of reactions, a skill essential for comprehending complex chemical processes.
This comprehensive guide will equip you with the knowledge and tools necessary to navigate reaction sequences with confidence, empowering you to identify functional groups, decipher reaction mechanisms, and predict regioselectivity and stereoselectivity. Through practice problems and real-world examples, you will hone your abilities and become a master of reaction sequence analysis.
1. Introduction
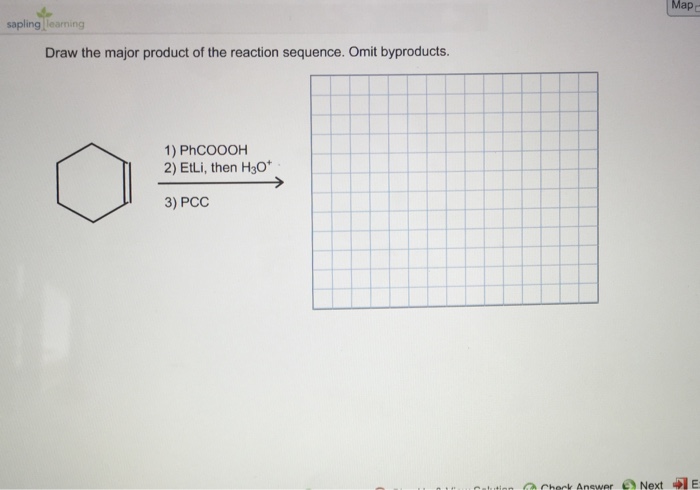
A reaction sequence is a series of chemical reactions that occur in a specific order. The major product of a reaction sequence is the product that is formed in the greatest amount. Drawing the major product of a reaction sequence is important because it allows chemists to predict the outcome of a reaction and to design synthetic strategies.
2. Identifying Functional Groups
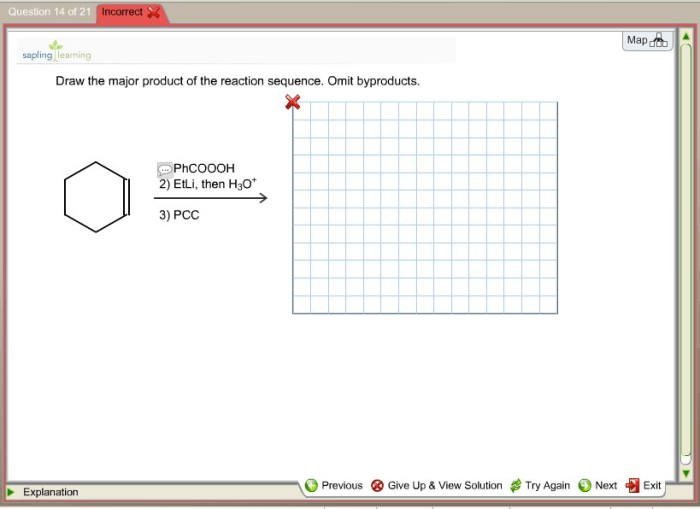
Functional groups are groups of atoms that have characteristic chemical properties. Identifying functional groups is important in a reaction sequence because it allows chemists to predict the reactivity of a molecule. Common functional groups include alcohols, alkenes, aldehydes, and ketones.
3. Reaction Mechanisms
Reaction mechanisms are the detailed steps by which a reaction occurs. Understanding reaction mechanisms is important because it allows chemists to design new reactions and to improve the efficiency of existing reactions. Common reaction mechanisms include nucleophilic substitution, electrophilic addition, and elimination reactions.
4. Regioselectivity and Stereoselectivity: Draw The Major Product Of The Reaction Sequence. Omit Byproducts.
Regioselectivity is the preference for a reaction to occur at one site over another. Stereoselectivity is the preference for a reaction to occur with one stereochemistry over another. Regioselectivity and stereoselectivity are important because they allow chemists to control the outcome of a reaction.
5. Using Chemical Structures
Chemical structures are used to represent molecules and reactions. Arrows are used to indicate the flow of electrons in a reaction. Understanding how to use chemical structures is important because it allows chemists to communicate about reactions in a clear and concise way.
6. Practice Problems
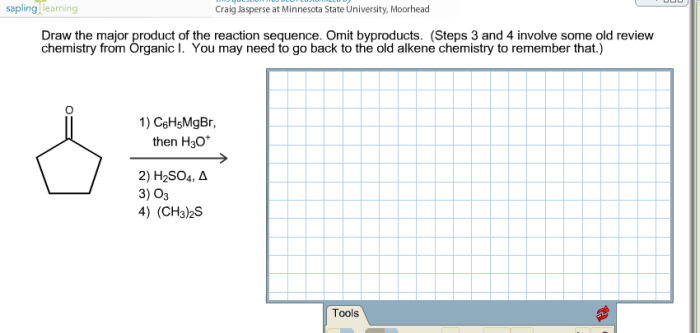
Practice problems are a great way to test your understanding of reaction sequences. Here are a few practice problems to get you started:
- Draw the major product of the following reaction sequence: “` CH3CH2Br + NaOH → CH3CH2OH CH3CH2OH + H2SO4 → CH3CH2OSO3H “`
- Draw the major product of the following reaction sequence: “` CH3CH=CH2 + HBr → CH3CHBrCH3 CH3CHBrCH3 + KOH → CH3CH=CH2 “`
Answers to Common Questions
What is the significance of identifying functional groups in reaction sequences?
Functional groups are the reactive centers of molecules, and their presence dictates the types of reactions that can occur. Identifying functional groups allows us to predict the possible products and mechanisms involved in a reaction sequence.
How do reaction mechanisms influence the outcome of a reaction sequence?
Reaction mechanisms provide a step-by-step account of how a reaction occurs, revealing the intermediates and transition states involved. Understanding reaction mechanisms enables us to predict the regioselectivity and stereoselectivity of a reaction, determining the orientation and spatial arrangement of the products.
What is the difference between regioselectivity and stereoselectivity?
Regioselectivity refers to the preference for a reaction to occur at a specific atom or position within a molecule, while stereoselectivity refers to the preference for a reaction to produce a specific stereoisomer (enantiomer or diastereomer). Both regioselectivity and stereoselectivity are crucial for controlling the outcome of reaction sequences.