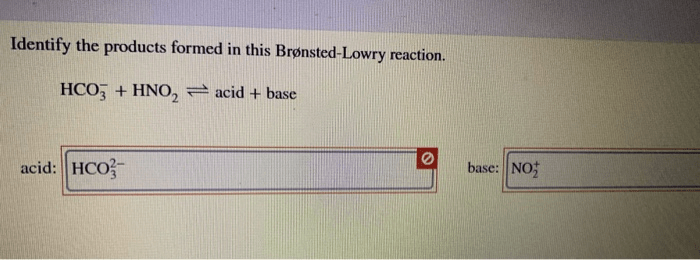
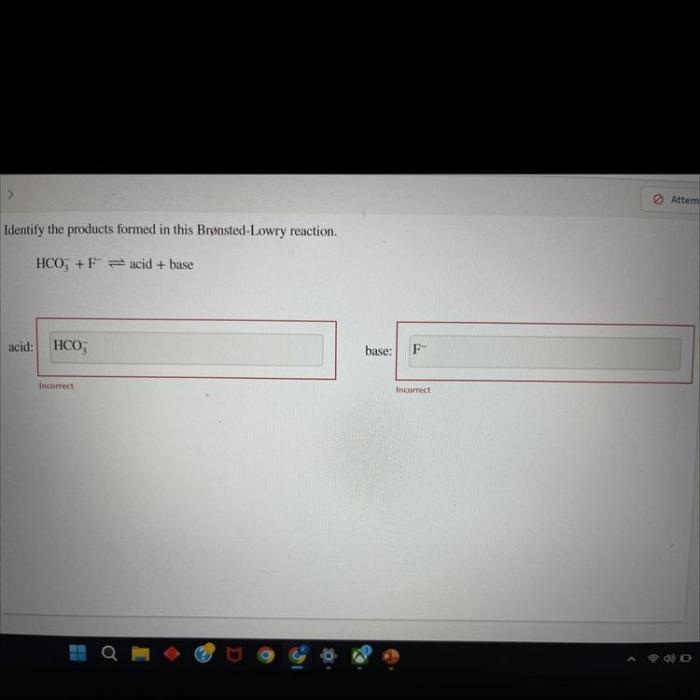
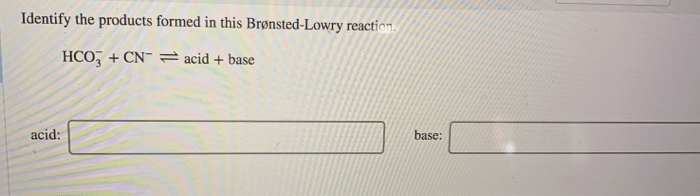
Identify the products formed in this brønsted-lowry reaction. – In the realm of chemistry, Brønsted-Lowry reactions play a pivotal role in understanding acid-base interactions. This guide delves into the intricacies of these reactions, empowering readers to identify the products formed and unravel their significance.
Brønsted-Lowry theory defines acids as proton donors and bases as proton acceptors. When an acid and a base react, they undergo a proton transfer, resulting in the formation of conjugate acid-base pairs. Understanding the steps involved in determining these products is essential for comprehending the mechanisms of these reactions.
Brønsted-Lowry Acid-Base Reactions
Brønsted-Lowry acid-base reactions are a type of chemical reaction that involves the transfer of a proton (H+) from an acid to a base. In this reaction, the acid donates a proton, and the base accepts it.
Acids are substances that can donate protons, while bases are substances that can accept protons.
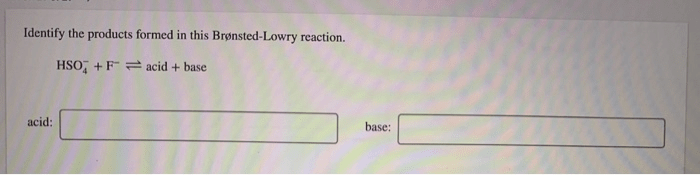
Product Identification
To identify the products formed in a Brønsted-Lowry reaction, we need to know the acid and the base involved in the reaction.
Once we know the acid and the base, we can use the following steps to identify the products:
- Write the balanced chemical equation for the reaction.
- Identify the acid and the base in the reaction.
- Determine which species is donating the proton and which species is accepting the proton.
- Write the products of the reaction.
Types of Brønsted-Lowry Reactions
There are two main types of Brønsted-Lowry reactions:
- Neutralization reactions
- Proton-transfer reactions
Neutralization reactions are reactions between an acid and a base that produce a salt and water.
Proton-transfer reactions are reactions between an acid and a base that produce a conjugate acid and a conjugate base.
Applications of Brønsted-Lowry Reactions, Identify the products formed in this brønsted-lowry reaction.
Brønsted-Lowry reactions are used in a variety of applications, including:
- The production of fertilizers
- The production of pharmaceuticals
- The treatment of water
- The preservation of food
Frequently Asked Questions: Identify The Products Formed In This Brønsted-lowry Reaction.
What are the key steps in identifying products formed in Brønsted-Lowry reactions?
1. Identify the acid and base involved in the reaction. 2. Determine the proton transfer that occurs. 3. Write the balanced chemical equation for the reaction. 4. Identify the conjugate acid-base pairs formed.
How can Brønsted-Lowry reactions be used in real-world applications?
Brønsted-Lowry reactions are employed in various fields, including: – Acid-base titrations for determining the concentration of acids or bases. – Buffer solutions for maintaining a stable pH in chemical and biological systems. – Industrial processes such as the production of fertilizers, dyes, and pharmaceuticals.