Moles and chemical formulas lab report answers provide a comprehensive understanding of the fundamental concepts of chemistry. This guide delves into the intricacies of moles, their significance, and their relationship with chemical formulas, empowering students with a solid foundation in this essential field.
Through engaging explanations and practical examples, this guide unravels the complexities of laboratory experiments, demonstrating how to determine the empirical formula of a compound. It also explores the applications of moles and chemical formulas, highlighting their importance in predicting the properties and behavior of compounds and determining the amounts of reactants and products in chemical reactions.
1. Moles and Chemical Formulas
An Introduction
Moles are a fundamental unit of measurement in chemistry. They are defined as the amount of substance that contains exactly 6.022 × 1023elementary entities (atoms, molecules, ions, or electrons).
Moles are used to express the amount of a substance in a convenient and meaningful way. They allow us to compare the quantities of different substances and to determine the stoichiometric ratios in chemical reactions.
The mole concept is closely related to the chemical formula of a compound. A chemical formula represents the elemental composition of a compound, indicating the types and proportions of atoms that make up the molecule.
Calculating the Number of Moles in a Sample
To calculate the number of moles in a given sample, we use the following formula:
n = m / M
where:
- n is the number of moles
- m is the mass of the sample (in grams)
- M is the molar mass of the substance (in grams per mole)
The molar mass of a substance is the sum of the atomic masses of all the atoms in its chemical formula.
Relationship Between Moles and Chemical Formulas
The relationship between moles and chemical formulas is that the number of moles of a substance is directly proportional to the number of formula units in a sample.
For example, if we have 1 mole of NaCl, we have 6.022 × 1023formula units of NaCl. This is because the chemical formula NaCl represents one formula unit of the compound.
2. Laboratory Experiment
Determining the Empirical Formula of a Compound

Purpose and Objectives
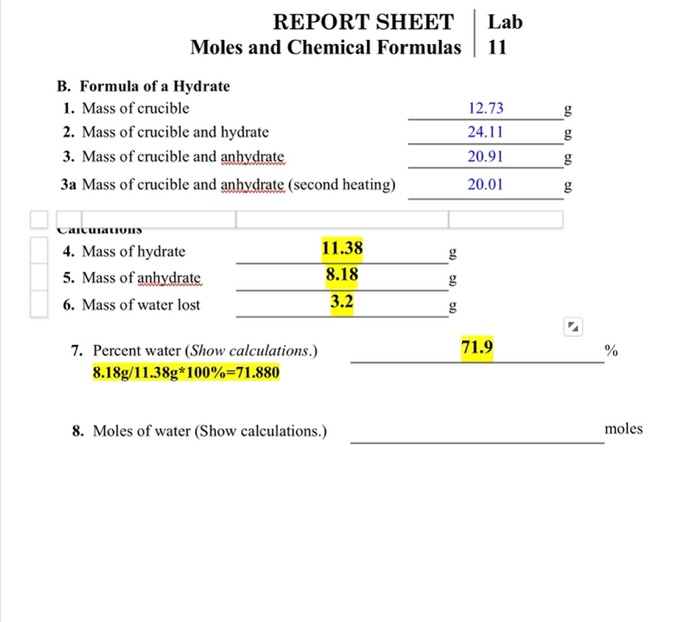
The purpose of this laboratory experiment is to determine the empirical formula of a compound. The empirical formula is the simplest whole-number ratio of the elements in a compound.
The objectives of this experiment are to:
- Measure the mass of a sample of the compound
- Decompose the compound into its constituent elements
- Measure the mass of each element
- Calculate the empirical formula of the compound
Experimental Procedure
The experimental procedure for this experiment is as follows:
- Weigh a sample of the compound.
- Decompose the compound into its constituent elements using a suitable method (e.g., heating, electrolysis, or acid-base reaction).
- Measure the mass of each element.
- Calculate the empirical formula of the compound using the following formula:
Empirical formula = XaY bZ c
where X, Y, and Z are the elements in the compound and a, b, and c are the number of atoms of each element in the empirical formula.
3. Example Calculations and Explanations

Example Calculation: Determining the Number of Moles in a Sample
Let’s say we have a sample of NaCl that weighs 58.44 grams. What is the number of moles of NaCl in the sample?
Using the formula n = m / M, we can calculate the number of moles as follows:
n = 58.44 g / 58.44 g/mol = 1 mol
Therefore, there is 1 mole of NaCl in the sample.
Example Calculation: Determining the Empirical Formula of a Compound, Moles and chemical formulas lab report answers
Let’s say we have a compound that decomposes into 4.00 g of carbon, 6.40 g of hydrogen, and 32.00 g of oxygen. What is the empirical formula of the compound?
First, we need to convert the mass of each element to moles:
- 4.00 g C × (1 mol C / 12.01 g C) = 0.333 mol C
- 6.40 g H × (1 mol H / 1.008 g H) = 6.35 mol H
- 32.00 g O × (1 mol O / 16.00 g O) = 2.00 mol O
Next, we need to find the simplest whole-number ratio of the moles of each element:
- C: 0.333 mol / 0.333 mol = 1
- H: 6.35 mol / 0.333 mol = 19
- O: 2.00 mol / 0.333 mol = 6
Therefore, the empirical formula of the compound is CH 19O 6.
4. Error Analysis and Sources of Uncertainty: Moles And Chemical Formulas Lab Report Answers
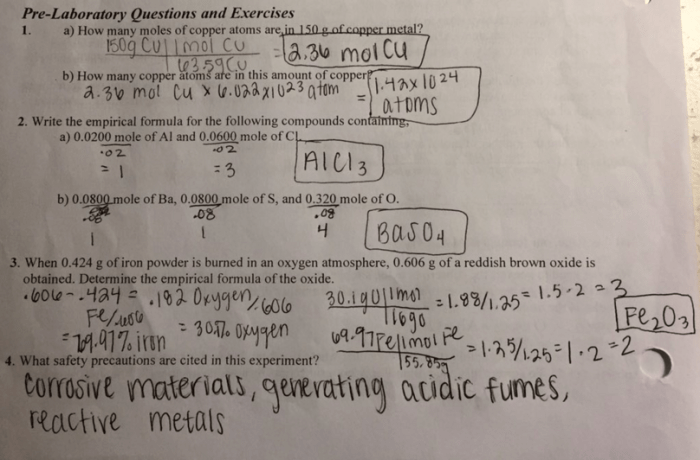
There are several potential sources of error in this laboratory experiment, including:
- Errors in weighing the sample
- Errors in measuring the mass of each element
- Errors in the decomposition of the compound
To minimize experimental errors, it is important to use accurate and precise equipment, to follow the experimental procedure carefully, and to repeat the experiment multiple times.
The accuracy of the results can be evaluated by comparing the empirical formula obtained from the experiment to the known empirical formula of the compound.
5. Applications of Moles and Chemical Formulas
Moles and chemical formulas have a wide range of applications in chemistry, including:
- Determining the amount of reactants and products in chemical reactions
- Predicting the properties and behavior of compounds
- Developing new materials and technologies
Moles are essential for understanding the quantitative aspects of chemistry, and chemical formulas provide a convenient way to represent the composition of compounds.
Commonly Asked Questions
What is the significance of moles in chemistry?
Moles serve as a bridge between the macroscopic and microscopic worlds, allowing chemists to relate the mass of a substance to the number of its constituent particles.
How do I calculate the number of moles in a given sample?
To determine the number of moles, divide the mass of the sample by its molar mass, which is the mass of one mole of the substance.
What is the relationship between moles and chemical formulas?
Chemical formulas represent the composition of a compound in terms of the number of atoms of each element present. Moles provide a quantitative measure of the amount of each element in a given sample.